How Soon Will We Have a Vaccine for the Coronavirus?

COVID Vaccine: How Far Away Is It?
By Anna Peterson Sanders, PharmD, pharmacist at Network Health
Originally published on 10/22/2020 at 9:00 a.m.
The devastation of the new coronavirus and resulting COVID-19 infection has been felt around the world. Because of this, it’s difficult to state how unifying the global pursuit of an immunization for the virus has been.
Backed by governments and private funding worldwide, the best scientific and biopharmaceutical minds have been working to bring us back to gathering and enjoying the activities we did before the outbreak without fear of a dangerous upper respiratory and vascular infection.
The big question on everybody’s mind is “how far away are we from having a vaccine?”
When will we have a COVID-19 vaccine?
Although it’s assumed that the first vaccine units will arrive in early 2021, that is – by no means – a certainty. There is still the question of which vaccine will become the one that helps save the world from the ever-increasing toll of the coronavirus.
COVID vaccine - options, updates and progress
There are many potential contenders for the title of the hero vaccine. In fact, over 200 COVID-19 vaccines are currently in development around the world. Yet, there are still plenty of questions.
- When will one be approved by the United States?
- Who will be the first to receive a vaccine?
- How effective will it be?
- What does long-term safety data look like?
Despite the many questions that the best and brightest are still looking to answer, information from the Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP) and Food and Drug Administration (FDA) can help us understand what to expect during this process of vaccine development.
Which COVID vaccines are currently undergoing clinical trials in the United States?
There are four vaccines currently underway with human clinical trials in the United States.
Of these four, two vaccine candidates, from manufacturers AstraZeneca and Johnson & Johnson, have been paused for safety reasons. The other two companies, Moderna and Pfizer/BioNTech, appear to be the frontrunners as they have entered into phase three trials, which is the last step prior to applying for FDA review and potential approval.
There are many similarities between these two vaccines and this chart references some key takeaways from the current status of these vaccine candidates.
Table 1. Active Phase Three United States Vaccine Candidates1, 2. 3, 4
|
Vaccine candidate |
mRNA-1273 |
mRNA-BNT162 |
|
Manufacturer |
Moderna TX, Inc. |
Pfizer, Inc./BioNTech |
|
Doses being studied |
Two doses (separated by 28 days) |
One or two doses (separated by 21 days) |
|
Route of administration |
Intramuscular (just like a flu shot is given) |
Intramuscular (just like a flu shot is given) |
|
Age group being studied |
18 and above |
12 and above |
|
Common side effects in early trials |
Pain, muscle aches and fatigue |
Fatigue, headache and muscle pain |
|
Manufacturer’s website |
What remains to be studied are certain vulnerable populations such as children, pregnant individuals and immunocompromised patients. Pfizer recently received FDA approval to expand their volunteer pool to include individuals as young as 12, making it the first U.S. trial to obtain approval for an age group this young.4
Future studies looking at safety and efficacy in higher-risk populations will likely be rolled out after the FDA has approved a vaccine.
Who will be the first group to receive the COVID vaccine?
Another common question about the COVID vaccine is “who will be first in line to receive it?”.
Analysts agree there will be a constrained supply upon FDA approval of the first vaccine. The manufacturing process will need to be ramped up to meet the demand. In the beginning stages, we will likely see a bottleneck phenomenon. While it’s nice to think about the vaccine being announced and then available the next day like a consumer electronic, it will take time for widespread availability.
For this reason, an administration strategy is crucial for meeting the public health needs of populations around the world.
The ACIP COVID-19 Vaccines Work Group has most recently proposed four groups to be targeted for the early phase of vaccination administration. The specifics of prioritization are still being worked out, but below are the groups identified for potential inclusion in early vaccine administration.
Table 2. Potential Groups Targeted for Early Phase of Vaccine Administration5
|
Group |
Examples |
|
Healthcare workers |
Hospital workers Long-term care facilities Pharmacies Home health care |
|
Essential workers |
Workers in education Law enforcement personnel Transportation services Food and agriculture workers |
|
Persons with high-risk medical conditions |
Cancer Chronic kidney disease Chronic obstructive pulmonary disease (COPD) Obesity Serious heart conditions Type 2 diabetes mellitus |
|
Older adults |
≥65 years old |
In ACIP’s monthly COVID-19 meetings, one common thread woven into each presentation is “safety, safety, safety.”
How will we know the forthcoming COVID vaccine is safe?
Safety is prioritized at each step of vaccine development as displayed in Figure 1 below. The COVID-19 vaccine will be tracked and analyzed long-term even after the vaccine has been approved by the FDA and administration begins in the general population.
Figure 1. The Vaccine Life Cycle: Safety at Every Phase5
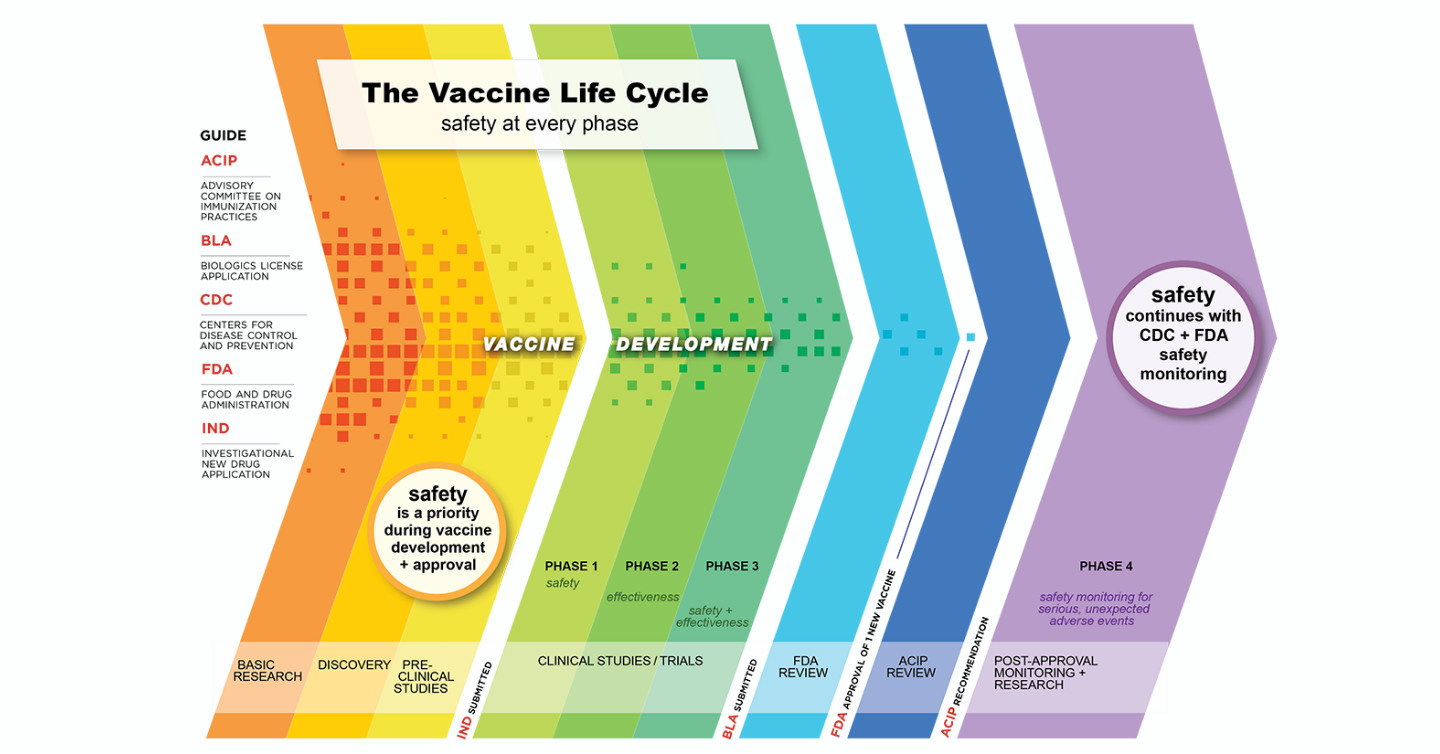
This post-approval surveillance approach is helpful in assessing safety related to certain areas that are more difficult to detect during phases one, two and three of the clinical trials, including the following.
- Discovering rare adverse reactions
- Seeing how the safety data compares between a clinical trial environment versus a real-world environment
- Determining safety in certain populations not included in the initial trials, such as those with certain pre-existing conditions
- Detecting side effects that may have a delayed onset
Post-approval safety surveillance extends beyond the drug manufacturer reporting safety data.
This phase includes many governmental agencies and is done independently from the drug manufacturers. The Vaccine Adverse Event Reporting System (VAERS), CDC’s Vaccine Safety Datalink (VSD), Veterans Affairs (VA), and the Centers for Medicare & Medicaid Services (CMS) are just a few of the agencies involved in a collaborative approach to monitoring the long-term safety of vaccines and other FDA-approved medications.7
Are COVID vaccine developers cutting any corners to get a vaccine out quickly?
No. In an unprecedented move, nine biopharmaceutical companies involved with development of COVID-19 candidate vaccines – including the four vaccines currently in phase three U.S. clinical trials, as mentioned above – signed a pledge to “help ensure public confidence in the rigorous scientific and regulatory process by which COVID-19 vaccines are evaluated and may ultimately be approved.”
Among their commitments, are the following.
- “To always make the safety and well-being of vaccinated individuals our top priority.
- To continue to adhere to high scientific and ethical standards regarding the conduct of clinical trials and the rigor of manufacturing processes.
- To only submit for approval or emergency use authorization after demonstrating safety and efficacy through a phase three clinical study that is designed and conducted to meet requirements of expert regulatory authorities such as the FDA.”8
So, not only are there existing processes and frameworks in place that require a focus on safety through a phased development approach, but the companies who are closest to having a vaccine have independently committed to not releasing a vaccine until it has met the same rigorous safety criteria as something like the flu shot or the vaccines we take for Hepatitis A and B.
What you can do until we have a COVID vaccine?
While a vaccine for COVID-19 is not yet available, there is a role we all can play in keeping ourselves and our loved ones as safe and healthy as possible.
- If interested in volunteering for any current or future COVID-19 clinical trials, consider filling out the screening registry at coronaviruspreventionnetwork.org.
- Follow the most up-to-date CDC guidelines for keeping yourself and your loved ones protected at gov/coronavirus/2019-nCoV/index.html
- Recommendations can change over time as we learn more about the virus, ways to protect ourselves and others and as treatments are investigated and developed
- Current recommendations include the below.9
- Washing your hands often
- Avoiding close contact (6 feet or less) with anyone who is sick or anyone that does not live in your household
- Wearing a mask around others in public and continuing to keep your distance from others since masks do not provide 100 percent protection from shedding the virus
- Take steps to keep yourself healthy
- Stay up-to-date with other vaccines, such as pneumonia and shingles vaccines
- Follow your doctor’s advice on controlling pre-existing diseases, like diabetes, COPD and heart disease. Uncontrolled conditions could increase complications if you contract COVID-19.
Finally, one of the biggest ways you can help, as we gear ahead for what the CDC has stated could be a very difficult winter, is to get the annual flu vaccine. By stopping in to a local pharmacy or making a short appointment with your personal doctor, you’re helping to keep hospitals and care facilities open and available for the expected influx of COVID-19 patients as we all move indoors for the cold months.
In fact, until we have a COVID vaccine, the flu shot is the best thing you can do to make sure those who wind up infected by the coronavirus are given the best shot possible at a quick and relatively easy recovery. Dr. Gretchen Wagner from Ascension gave us some reasons why the flu shot will be important this year in another blog we recently published on Grow in the Know. You can read it here.
Finally, your Network Health plan can assist you with preventive measures that can help stave off illness until we have a vaccine available for public administration. For more information about ways Network Health is working to keep communities in Wisconsin healthy and strong during this time, reach out to us today.
Also, be sure to bookmark this page, as we’ll be updating it monthly or upon the communication of significant events in the development of a COVID vaccine.
References
- Miller, J. mRNA-1273 Clinical Development Program. Oral presentation at: Advisory Committee on Immunization Practices; August 28, 2020; Atlanta, GA.
- Oliver, S. COVID-19 vaccines: Work Group interpretations. Oral presentation at: Advisory Committee on Immunization Practices; August 28, 2020; Atlanta, GA.
- Kitchin, N. Pfizer/BioNTech COVID-19 mRNA vaccine. Oral presentation at: Advisory Committee on Immunization Practices; August 28, 2020; Atlanta, GA.
- Aubrey, A. Will Kids Get A COVID-19 Vaccine? Pfizer To Expand Trial To Ages 12 And Up. Nation Public Radio Website. npr.org/sections/health-shots/2020/10/13/923248377/will-kids-get-a-covid-19-vaccine-pfizer-to-expand-trial-to-ages-12-and-up. Published October 13, 2020. Accessed October 19, 2020.
- Dooling, K. COVID-19 vaccine prioritization: Work Group considerations. Oral presentation at: Advisory Committee on Immunization Practices; August 28, 2020; Atlanta, GA.
- Overview, History, and How the Safety Process Works. Centers for Disease Control and Prevention Website. cdc.gov/vaccinesafety/ensuringsafety/history/index.html. Updated July 1, 2020. Accessed August 26, 2020.
- Shimabukuro, T. COVID-19 vaccine safety monitoring. Oral presentation at: Advisory Committee on Immunization Practices; August 28, 2020; Atlanta, GA.
- COVID-19 Vaccine Maker Pledge. International Federation of Pharmaceutical Manufacturers & Associations Website. ifpma.org/resource-centre/covid-19-vaccine-maker-pledge/. Published September 8, 2020. Accessed October 19, 2020.
- How to Protect Yourself & Others. Centers for Disease Control and Prevention Website. cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Updated July 31, 2020. Accessed August 27, 2020.



